Insights+: EMA Marketing Authorization of New Drugs in June 2024
Shots:
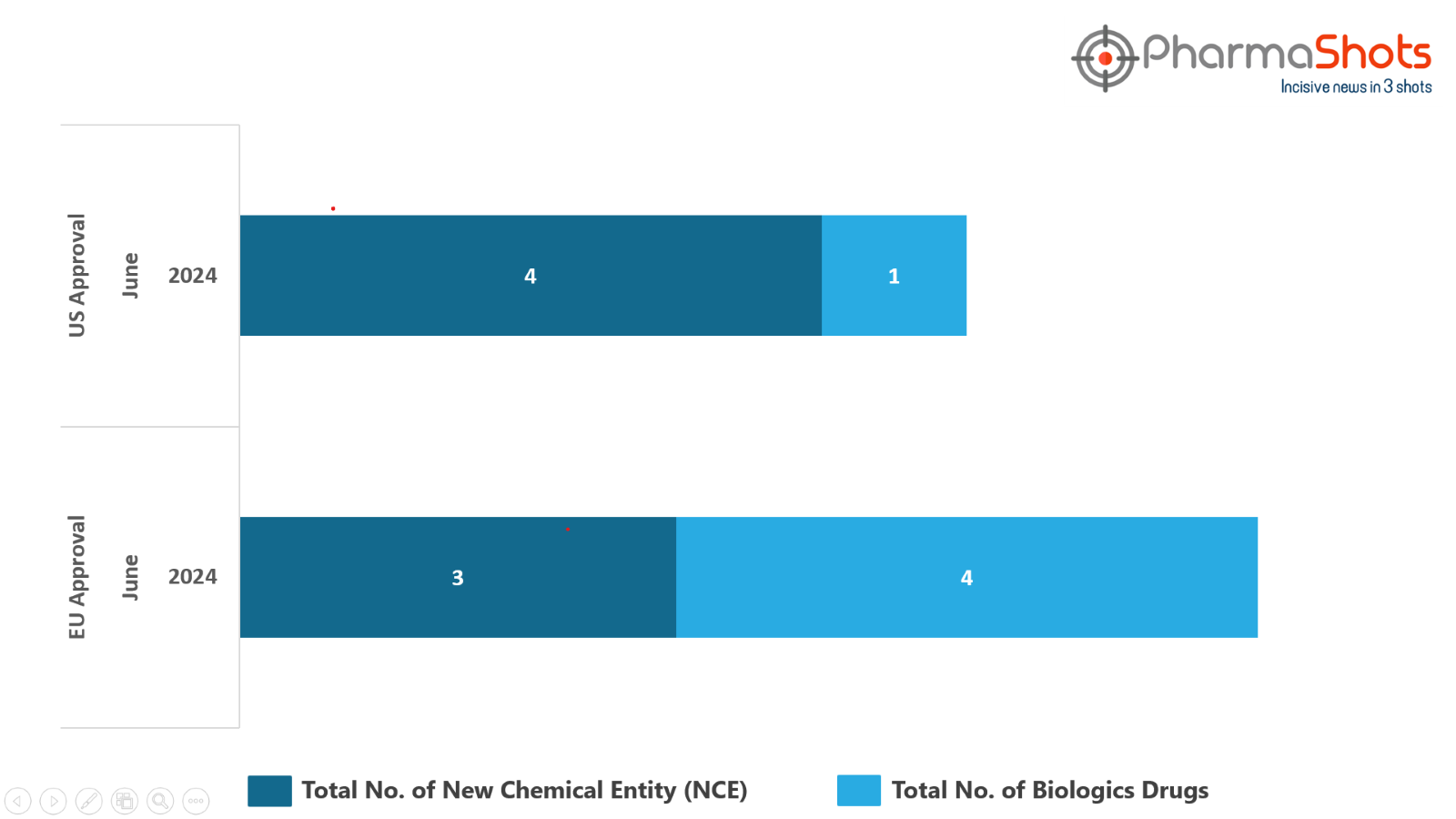
- The EMA granted Positive Opinion to 4 Biologics and 3 New Chemical Entities in June 2024, leading to treatments for patients and advances in the healthcare industry
- The major highlighted drugs were Merck’s Winrevair to treat Pulmonary Arterial Hypertension (PAH) and Roche’s PiaSky for Paroxysmal Nocturnal Haemoglobinuria (PNH)
- PharmaShots has compiled a list of 5 drugs that have been granted positive opinion by the EMA’s CHMP
Product Name: PiaSky
Active ingredient: Crovalimab
Company: Roche
Date: June 27, 2024
Disease: Paroxysmal Nocturnal Haemoglobinuria
Shots:
- The CHMP’s positive opinion of PiaSky for PNH adults & adolescents (≥12yrs., 40kg) was based on 3 P-III trials incl. COMMODORE 2 study of PiaSky (SC, Q4W) vs eculizumab (IV, Q2W) in patients not treated with C5 inhibitor; COMMODORE 1 study in subjects switched from C5 inhibitor & COMMODORE 3 study in Chinese subjects new to C5 inhibitor
- COMMODORE 2 study showed that the drug was superior, well-tolerated & attained disease control with 78% vs 80% AEs
- PiaSky (C5 protein inhibiting mAb) is currently being studied under 5 P-III clinical evaluations & 3 earlier phase studies to treat complement-mediated diseases such as PNH, atypical haemolytic uremic syndrome & sickle cell disease
Product Name: mResvia
Active ingredient: mRNA-1345
Company: Moderna
Date: June 27, 2024
Disease: Lower Respiratory Tract Disease
Shots:
- The CHMP’s positive opinion of mRESVIA to prevent lower respiratory tract disease (LTRD) due to RSV infection based on the P-III (ConquerRSV) study in adults (n=37,000; ≥60yrs.)
- Primary analysis (3.7mos. median follow-up) showed vaccine efficacy (VE) of 83.7%, published in the NEJM. Supplementary analysis (8.6mos. median follow-up) showed sustained VE of 63.3% against RSV-LRTD incl. ≥2 symptoms with VE of 74.6% & 63% with ≥2 & ≥3 symptoms, respectively
- In addition, the vaccine received the US FDA’s approval in May 2024 for the same. Furthermore, the company has filed MAA to other global authorities
Product Name: Winrevair
Active ingredient: Sotatercept
Company: Merck
Date: June 27, 2024
Disease: Pulmonary Arterial Hypertension
Shots:
- The CHMP has granted positive opinion to Winrevair combined with other therapies to treat PAH. EC’s decision is anticipated in Q3’24, applicable across EU, Iceland, Liechtenstein & Norway
- The opinion was based on the P-III (STELLAR) study evaluating the safety & efficacy of Winrevair (target dose 0.7mg/kg; n=163) or PBO (n=160) + stable background therapy in PAH patients (N=323)
- Winrevair improved 6-minute walk distance (1EP) as well as reduced the death risk from any cause & PAH clinical worsening events (2EPs). Data was published in the NEJM
Product Name: Ordspono
Active ingredient: Odronextamab
Company: Regeneron
Date: June 28, 2024
Disease: Follicular Lymphoma or Diffuse Large B-Cell Lymphoma
Shots:
- The CHMP has granted a positive opinion for conditional marketing authorization of odronextamab (CD20xCD3 bispecific Ab) to treat r/r FL or r/r DLBCL post ≥2L of therapies, with the EC’s decision anticipated in the upcoming mos.
- The opinion was based on the P-I (ELM-1) & pivotal P-II (ELM-2) studies, showing strong durable response rates & safety in r/r FL or r/r DLBCL adults
- Regeneron is assessing odronextamab alone & in combinations as earlier lines of therapies for lymphomas, incl. the registrational ELM-1 & ELM-2 trial, P-III (OLYMPIA) study for B-NHLs and early-stage studies with CT-free combinations
Product Name: Balversa
Active ingredient: Erdafitinib
Company: Janssen
Date: June 28, 2024
Disease: Metastatic Urothelial Carcinoma
Shots:
- The CHMP has granted a positive opinion to Balversa monotx. (QD, oral) to treat inoperable mUC adults with susceptible FGFR3 mutations & previously treated with at least 1L of therapy with PD-1/PD-L1 inhibitor
- The opinion was based on the arm 1 of P-III (THOR) trial assessing the safety & efficacy of erdafitinib (n=136) vs CT (n=130) for mUC. Trial was halted, in Jun 2023, at interim analysis with patients randomizing on CT & with an option of crossing over to erdafitinib
- The study showed an mOS of 12.1mos. vs 7.8mos. at the data cut off, improved mPFS of 5.6mos. vs 2.7mos. and ORR of 35.3% vs 8.5%
Note:
According to the EMA’s June 2024 list, the following drugs received the CHMP’s positive opinion; however, no PR was available:
- Tauvid [flortaucipir (18F)]
- Eurneffy (epinephrine)
Related Post: Insights+: EMA Marketing Authorization of New Drugs in May 2024




